REACH
To ensure the health and safety of a population, many countries have implemented chemical registration regulations to track and evaluate the impact of the chemicals that are being manufactured, imported, and used. REACH is the European Regulation (EC) No 1907/2006, on the Registration, Evaluation, Authorisation and restriction of Chemicals adopted to improve the protection of human health and the environment from the risks that can be posed by chemicals. It aims to enhance the competitiveness of the European Union (EU) chemicals industry and promote alternative methods for the hazard assessment of substances in order to reduce the number of tests on animals.
REACH entered into force in 2007 and shifted the responsibility from public authorities to industry with regard to assessing and managing the risks posed by chemicals and providing appropriate safety information for their users. To find out more about the REACH regulatory website consult the ECHA website.
Hydrocarbon Substances and REACH
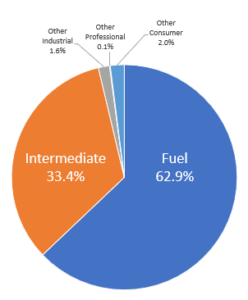
In 2022, 889 million tonnes of hydrocarbon substances were registered for REACH (manufactured or imported into the EU). 96% of this tonnage is currently used as either fuel or as an intermediate.
During the Registration phase, Concawe had a formal role as the Substance Information Exchange Forum (SIEF) Formation Facilitator for all registered hydrocarbon substances and is now managing the Evaluation phase on behalf of the SIEFs participants. The SIEF allows to share data generated for REACH (by Concawe or one or more of the registrants) amongst all registrants of the same substance, thus avoiding duplication of effort. The Commission implementing regulation on data sharing ((EU) 2016/9) allows a fair share of the cost incurred in generating data for REACH to be shared amongst co-registrants (post-SIEF). Currently, there are around 4,100 active registrations of Concawe hydrocarbon substances.
As SIEF Coordinator, Concawe provides licence agreements for hydrocarbon substances. Should you intend to register such a substance, please send an e-mail to the SIEF Team of Concawe (admin@super-sief.eu) mentioning:
- your Legal Entity Name as indicated in REACH-IT;
- the UUID number of your Legal Entity in REACH-IT;
- the e-mail address that will become the SIEF Space account user.
Concawe aims at identifying hydrocarbon substances endpoints for which further work is needed, following ECHA’s evolving guidances, to inform proper regulatory assessment of all Concawe hydrocarbon substances. The Evaluation strategy aims to maximise assessment efficiency in terms of time, animal welfare, and cost, while not underestimating potential risks to human health and the environment.
Concawe updates their dossier on an annual basis. For more information about the dossier update, click here.
Substance Identity and Composition
Hydrocarbon substances in Concawe’s portfolio are of Unknown or Variable composition, Complex reaction products or Biological materials (UVCB). The precise identity and composition of every constituent is most of the time unknown and the composition may vary across samples of the same substance.
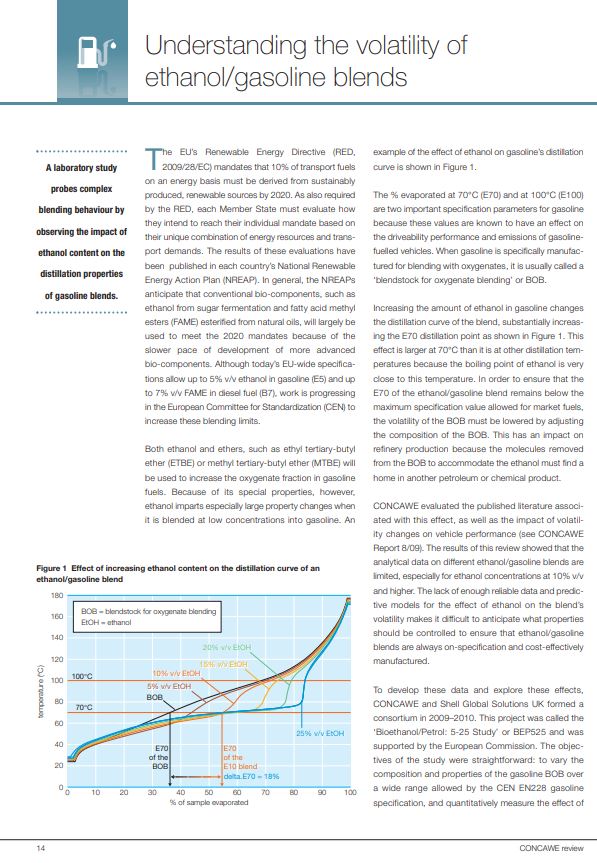
The biggest challenge in applying REACH to hydrocarbon substances is to account for their UVCB nature. The complexity of UVCB substances, their variability and their large number of potential constituents (which can reach over a million of molecules), makes it is impossible to determine the precise chemical composition to the level of each constituent for the majority of hydrocarbon substances. For many applications of hydrocarbon products, a detailed chemical composition is not necessary, because industry practice is to manufacture and market hydrocarbon substances according to physicochemical parameters specified in European Standards. Different samples from the same process in a refinery will show some variability in detailed composition, whilst still remaining within the specifications that identify the substance.
Read-across and substance similarity
Vertebrate animal studies have long served as the backbone in human health toxicological assessments. They are also central to some endpoints in eco-toxicological registrations. As one of the pillars of REACH legislation is to avoid unnecessary animal studies, registrants must consider appropriate New Approach Methodologies (NAMs) to fulfil information requirements for testing each substance and avoid unnecessary animal studies. Read-across (RA) is a commonly used alternative approach for data gap-filling. RA involves the use of relevant information from analogous “source” substances to predict the properties of the “target” substances under consideration. Relevant information requires primarily structural or compositional characterisation, as well as physical-chemical properties and biological activity profiles. In addition to reducing animal use, the proper application of RA can improve the quality of the hazard assessment by bringing into consideration the weight of evidence of closely related substances and reducing the time required to provide compliant information for a substance.
Further information about Hydrocarbon space mapping to support gas oil read-across for human health hazard assessment can be found in the Concawe Review Volume 31.
Human Health
For the 2010 REACH registration deadline, the Concawe dossiers were completed with the required toxicological information on petroleum substances. Specific endpoint requirements were either fulfilled with actual data on the substance itself or by application of structural read-across (RA, discussed above) to relevant data on related substances.
To further comply with the evolving REACH legislation and granularity of data required by ECHA, new data is generated on some toxicity endpoints (incl. genotoxicity, repeated dose toxicity and/or reproductive toxicity) for several Concawe petroleum product categories.
For instance, reproductive toxicity data is a standard data requirement in REACH, comprising of two endpoints: i) Pre-Natal Developmental Toxicity (PNDT) and ii) fertility (more recently by an Extended One-Generation Reproductive Toxicity Study (EOGRTS)). As permission from ECHA is required before conducting a higher-tier toxicity test in vertebrate animals to satisfy these endpoints, Concawe submits testing proposals to conduct PNDT and EOGRT studies. The current paradigm expects all testing proposals for non-CMR substances within the Concawe portfolio to be submitted by 2030.
This is part of the overall Concawe REACH strategy for Human Health, which consists also of reducing animal testing and the development and use of new alternative methodologies to assess biological coherence between substances in categories and to further strengthen RA justifications.
As such, a multi-year research project was initiated to bolster the read-across argument with detailed structural and biological data. A first step has been to better understand the variable composition within each substance and category to be able to identify representative samples for testing. The central tenet to the toxicology hypothesis is that reproductive toxicity is correlated to, and a product of, the quantity of 3-7 ring polycyclic aromatic hydrocarbons (PAHs) in petroleum substances. However, in order to not underestimate the hazards, the tested samples should also be representative of the whole hydrocarbon space of the considered substances and categories.
A second step is to support biological similarity. This is done with standard shorter-term animal testing, or bridging studies. Results from these oral 28-day screening studies (following the OECD 422 protocol) are compared to allow Concawe to ‘bridge’ the substances and apply results from the source substance that will be subject to higher-tier testing, to the target substances across the category.
The future hope is to apply in-vitro biological techniques (such as the Cat-App project previously led by Concawe) and omics technologies (e.g. metabolomics, that aim at the collective characterization and quantification of pools of small biological molecules in a given organism) through the lens of the more classical OECD 422 results with hydrocarbon composition data to minimise the need of future animal testing.
Environment
For successful substance registration under REACH and appropriate classification under CLP, several types of environmental information are needed:
- Data on ecotoxicity and fate to ensure accurate classification under CLP;
- An environmental risk assessment to determine whether the specific uses of a substance can be considered safe for the environment under REACH;
- An assessment of persistence, bioaccumulation, mobility, and toxicity to determine whether the substance fulfils the criteria of PBT/vPvB under REACH and CLP or PMT/vPvM under CLP;
- An assessment of endocrine disruption to determine if the substance fulfils the ED criteria under CLP.
Due to the complex nature of hydrocarbon substances, the hydrocarbon block method (HCBM) was developed by Concawe to evaluate the behaviour of these substances when released into the environment. The HCBM divides a hydrocarbon substance into blocks of related constituents on the basis of chemical classes (e.g. n-paraffins, isoparaffins, naphthenics, and aromatics) and carbon number distribution. The separation of hydrocarbon substance constituents to permit the HCBM is typically done using 2-dimensional gas chromatography coupled with a flame ionization detector.
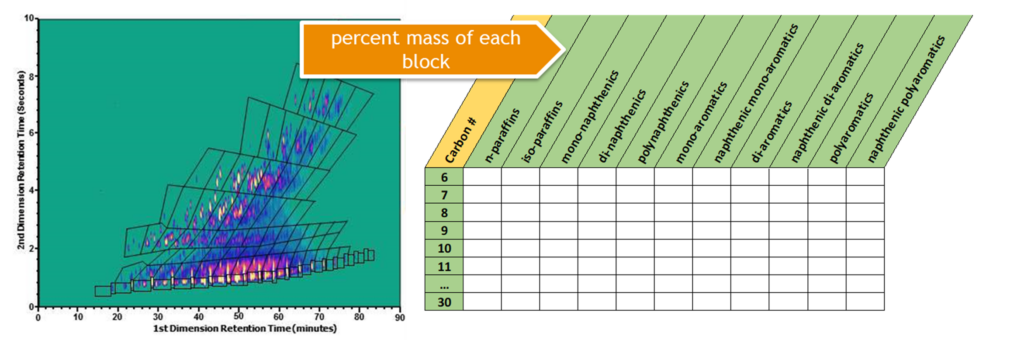
The fate and effects of these blocks can then be modelled to assess the environmental risks posed by a substance and its uses. This environmental risk assessment is performed using a tool known as Petrorisk.
In addition to the risk assessment which is conducted using Petrorisk, Concawe has filled the information requirements in the registration dossiers for REACH and for classification and labelling by compiling experimental ecotoxicity data for hydrocarbon substances and using models, like the EpiSuite models and Concawe developed Petrotox, which simulates aquatic toxicity testing of hydrocarbon substances based on compositional information using similar principles to Petrorisk.
Under REACH, the PBT/vPvB assessment must consider all relevant constituents and impurities of a substance composition. This poses a significant challenge for UVCBs, which contain a large number of constituents. Concawe’s PBT/vPvB assessment is based on hydrocarbon blocks and utilises a combination of experimental data for individual hydrocarbons and model data for a library of 16,000 representative constituents. This assessment is documented in the Concawe PBT report.
Concawe undertakes multiple research projects to generate further experimental data to support the assessment of hydrocarbon substances. These include novel approaches to assess the biodegradation of hydrocarbons, bioaccumulation in terrestrial organisms, methods to evaluate endocrine disruption, and approaches to evaluate complex UVBC substances.
Concawe also actively engages with regulatory initiatives, like the PetCo Working Group, PBT Expert Group, and an ED Expert Group to work collaboratively with ECHA and Member State Competent Authorities (MSCAs) to address concerns about the assessment of hydrocarbon substances.
Product Stewardship
The objective of Product Stewardship is to guarantee the development of a responsible, competitive and sustainable chemical industry in line with the principles and objectives of the European REACH and CLP legislations, and of relevant national regulations.
In this mainstream, Concawe’s Product Stewardship teams help companies to assess the hazards and risks of their products and manage accordingly the impact on the health and safety of workers and consumers, and the environment, derived from their use, handling or disposal throughout their life-cycle.
Concawe develops Product Stewardship guidelines with the aim of supporting companies to develop their own Product Stewardship management, complying with EU chemicals regulations (principally REACH and CLP) and standards.
The Product Stewardship Management Group and its Special Task Force provide guidance, best practices and reports to build up efficient management of safe handling and use of Concawe-related products.
Hazardous substances are subject to the CLP notification requirement. Articles 39 – 42 of the CLP Regulation (EC) No 1272/2008 require manufacturers and importers to notify the classification and labelling of hazardous substances (as such, in mixtures or in articles). The notification is required in a specified format and needs to be updated according to the latest legislation developments (ATPs to CLP). A Concawe working group is following the evolution of the legislative requirements and the format updates for the submission.
The Classification and Labelling of Petroleum Substances report is regularly updated to support companies to be compliant with the EU regulation on the Classification, Labelling and Packaging of substances and mixtures (CLP).
As a result of the many changes arising from the different revisions of Annex II of REACH and transition periods for implementing the guidance, Concawe Product Stewardship teams issue and regularly update the Guidance on the compilation of Safety Data Sheets (SDS) for Petroleum Products to support companies in the compilation of their respective SDS.
Trading is not mentioned in REACH regulation although it is a well-established activity in most fuels manufacturers companies. With the aim of facilitating the compliance of the trading activities to REACH and CLP Regulations, a Product Stewardship sub-group develops supporting documents for companies on the definition of rule and responsibilities in trading, imports and warehousing.