Dossier Updates
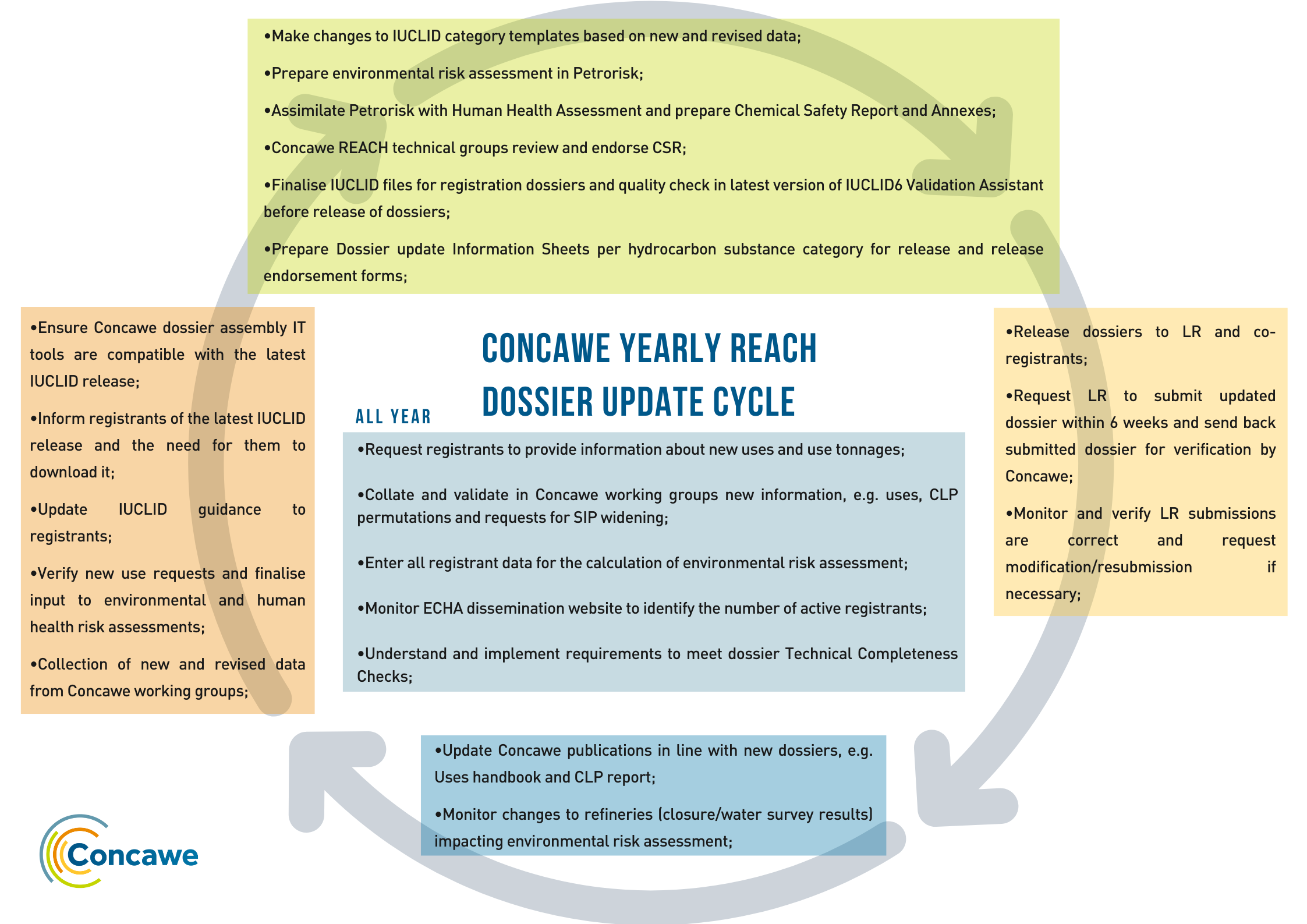
REACH requires registrants to keep their substance dossiers up to date. In addition to the mandatory updates required in response to ECHA decisions, Concawe has a comprehensive process in place to voluntarily update their hydrocarbon substance REACH dossiers on an annual basis.
Changes in the joint submission information, including new uses, classification and labelling permutations, substance identity profiles, revised and new hazard data, together with changes to accommodate ECHA requirements in dossier technical completeness checks and IUCLID versions, are addressed by Concawe with input from the member company experts. These ensure a continued high quality of its dossiers according to the latest scientific insights and new information available. Revised dossiers are released to all hydrocarbon substance registrants supported by Concawe on an annual basis, who then merge the revised dossier from Concawe with their own dataset to submit an updated dossier in REACH IT.
These regular dossier updates are in line with the requirements of the new Implementing Regulation (EU) 2020/1435, which sets out the timelines by which all registrants are required to update their registration dossiers when new information becomes available. To facilitate fulfilment of the Regulation, Concawe has recently issued guidance to over 4000 registrants of the over 160 hydrocarbon substances it provides the joint submission dossier and supports as SIEF facilitator. This guidance explains for lead and coregistrants for each type of information change, whether this is managed directly by the registrant or via Concawe, the process and timings for updates.
The Concawe annual process of dossier updates is supported by a Concawe team of experts dedicated to REACH compliance, specialists from across the membership of fuel manufacturers in Europe and dedicated consultants. These are dealing with the provision, review and application of new data, including that from its extensive science and regulatory programs supporting substance identity and physical properties, environmental & human health hazard, exposure and risk assessments, and product stewardship (including classification & labelling and poison centre requirements). In addition, an IUCLID team supports the automation of dossier assembly, and a SIEF team to support the release of the dossiers, associated cost-sharing and registrant communications. The dossier update coordination team maintains good contact with ECHA to ensure that new developments in technical completeness checks are fully implemented in the annual update.
Registrants benefit from this comprehensive annual review and update with high quality and consistent data, maximising the value of new data and helping the regulatory process overall.